API and Intermediate
Etoricoxib
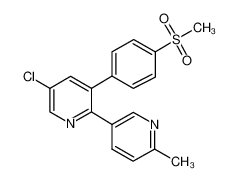
Product Name:Etoricoxib
Synonyms:Nucoxia;UNII-WRX4NFY03R;5-chloro-3(methylsulfonyl)phenyl-2-((4-methyl)-3-pyridyl)-pyridine;
5-Chloro-6'-methyl-3-(4-(methylsulfonyl)phenyl)-2,3'-bipyridine;Tauxib;Algix;5-chloro-3-(4-(methylsulfonyl)phenyl)-2-(2-methyl-5-pyridinyl)pyridine;5-chloro-3-(4-(methylsulfonyl)phenyl)-2-(Methyl-5-pyridinyl) pyridine;Arcoxia;Etoricoxibe;2-(6-methylpyrid-3-yl)-3-(4-methylsulfonylphenyl)-5-chloropyridine;
5-chloro-2-(6-methylpyridin-3-yl)-3-(4-methylsulfonylphenyl)pyridine;[14C]-Etoricoxib;3-(4-methylsulfonylphenyl)-2-(2-methyl-5-pyridyl)-5-chloro-pyridine;MK-0663;MK-663;
CAS:202409-33-4
MF:C18H15ClN2O2S
MW:358.84200
Appearance & Physical State:off-white powder
Density:1.298 g/cm3
Boiling Point:510ºC at 760 mmHg
Melting Point:134-135ºC
Flash Point:262.2ºC
Refractive Index:1.6
Vapor Pressure:5.17E-10mmHg at 25°C
Description:Etoricoxib (Arcoxia) is a selective COX-2 inhibitor from Merck & Co. Currently it is approved in more than 80 countries worldwide but not in the US, where the Food and Drug Administration (FDA) has required additional safety and efficacy data for etoricoxib before it will issue approval.
It is a selective COX-2 inhibitor with anti-inflammatory, analgesic and antipyretic effects. It is suitable for the treatment of symptoms and signs of acute and chronic osteoarthritis. It can also treat acute gouty arthritis.





